在今天一个相当具有指标意义的发表中,世界卫生组织(WHO)核可了世界第一款疟疾疫苗「RTS,S」,或名为 Mosquirix。WHO 主要是将它推荐给非洲及其他疟疾频发区域的儿童使用,该疫苗在防止感染上有大约 39% 的效力,而在避免重症上则是有约 29% 的效力。就数字来看,这似乎并不高,但由于疟疾是透过蚊子来传播,因此还能搭配蚊帐、杀虫剂和环境清洁、消除积水容器等方式,达到有效控制疟疾散布的目的。WHO 预期如果能大范围施打疫苗的话,每年可以多救下数万条人命 —— 目前每年都有多达 26 万名非洲儿童死于疟疾。
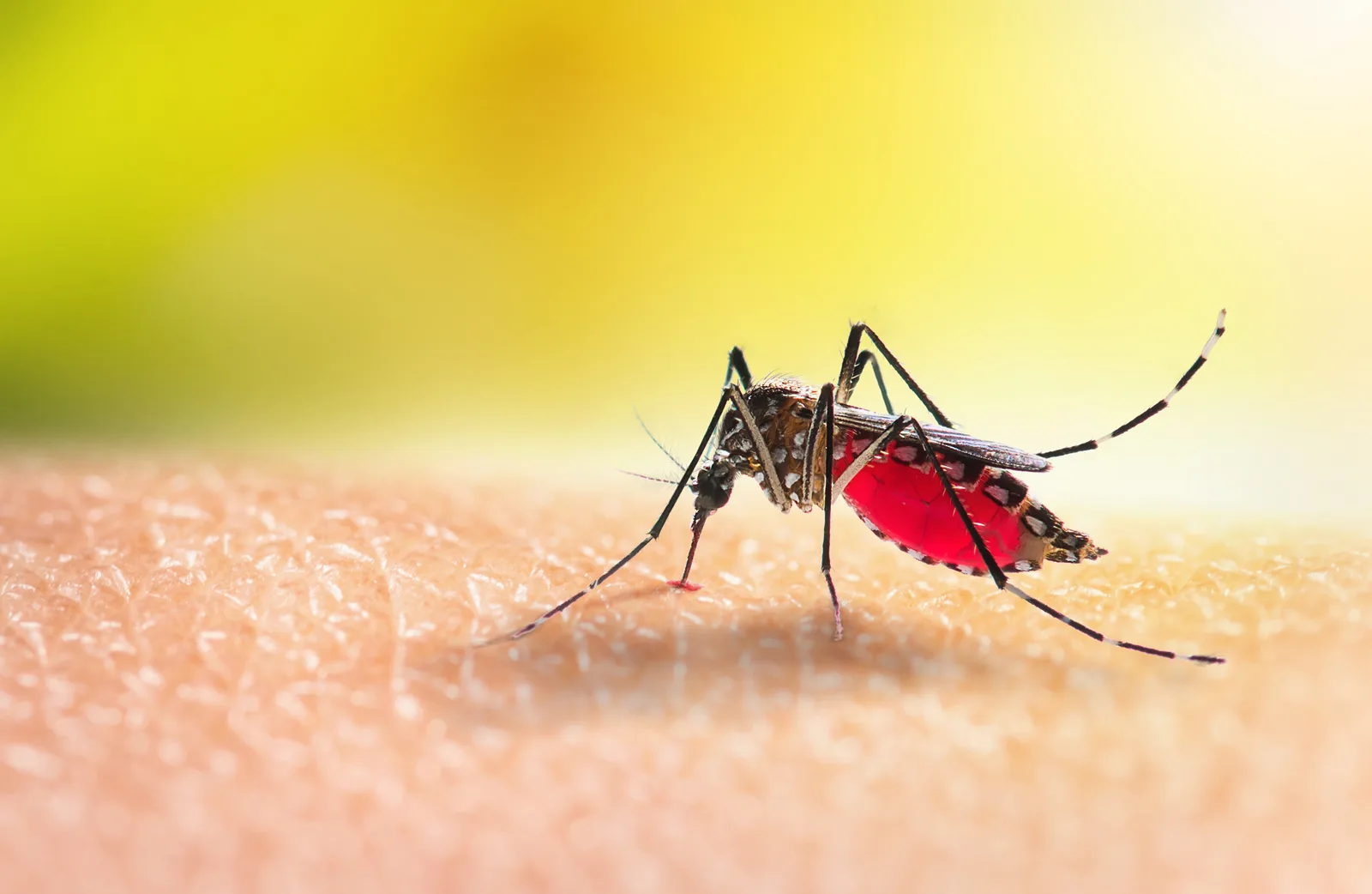
WHO 称 RTS,S 部署简单、使用安全,并且相对便宜。疫苗背后的药厂 GlaxoSmithKline(GSK)表示,每年将以不超过生产成本 5% 的低价,提供至少 1500 万剂疫苗供 WHO 调度,并且两方也在寻找其他合作伙伴或经费来源。
RTS,S 预期只是个开端而已,在 mRNA 技术通过此次 COVID-19 疫情获得充份的实证后,未来以 mRNA 技术为基础开发的新一代疟疾疫苗可以加速上市,其中牛津大学的 R21 疫苗有着高达 77% 的效力,并且目前的测试都显示它是安全的。这意味着人类朝向终于终结疟疾这个古老的疾病,又更进了一步了吧。
WHO recommends groundbreaking malaria vaccine for children at risk
Historic RTS,S/AS01 recommendation can reinvigorate the fight against malaria
The World Health Organization (WHO) is recommending widespread use of the RTS,S/AS01 (RTS,S) malaria vaccine among children in sub-Saharan Africa and in other regions with moderate to high P. falciparum malaria transmission. The recommendation is based on results from an ongoing pilot programme in Ghana, Kenya and Malawi that has reached more than 800 000 children since 2019.
“This is a historic moment. The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control,” said WHO Director-General Dr Tedros Adhanom Ghebreyesus. “Using this vaccine on top of existing tools to prevent malaria could save tens of thousands of young lives each year.”
Malaria remains a primary cause of childhood illness and death in sub-Saharan Africa. More than 260 000 African children under the age of five die from malaria annually.
In recent years, WHO and its partners have been reporting a stagnation in progress against the deadly disease.
“For centuries, malaria has stalked sub-Saharan Africa, causing immense personal suffering,” said Dr Matshidiso Moeti, WHO Regional Director for Africa. “We have long hoped for an effective malaria vaccine and now for the first time ever, we have such a vaccine recommended for widespread use. Today’s recommendation offers a glimmer of hope for the continent which shoulders the heaviest burden of the disease and we expect many more African children to be protected from malaria and grow into healthy adults.”
WHO recommendation for the RTS,S malaria vaccine
Based on the advice of two WHO global advisory bodies, one for immunization and the other for malaria, the Organization recommends that:
WHO recommends that in the context of comprehensive malaria control the RTS,S/AS01 malaria vaccine be used for the prevention of P. falciparum malaria in children living in regions with moderate to high transmission as defined by WHO. RTS,S/AS01 malaria vaccine should be provided in a schedule of 4 doses in children from 5 months of age for the reduction of malaria disease and burden.
Summary of key findings of the malaria vaccine pilots
Key findings of the pilots informed the recommendation based on data and insights generated from two years of vaccination in child health clinics in the three pilot countries, implemented under the leadership of the Ministries of Health of Ghana, Kenya and Malawi. Findings include:
- Feasible to deliver: Vaccine introduction is feasible, improves health and saves lives, with good and equitable coverage of RTS,S seen through routine immunization systems. This occurred even in the context of the COVID-19 pandemic.
- Reaching the unreached: RTS,S increases equity in access to malaria prevention.
- Data from the pilot programme showed that more than two-thirds of children in the 3 countries who are not sleeping under a bednet are benefitting from the RTS,S vaccine.
- Layering the tools results in over 90% of children benefitting from at least one preventive intervention (insecticide treated bednets or the malaria vaccine).
- Strong safety profile: To date, more than 2.3 million doses of the vaccine have been administered in 3 African countries – the vaccine has a favorable safety profile.
- No negative impact on uptake of bednets, other childhood vaccinations, or health seeking behavior for febrile illness. In areas where the vaccine has been introduced, there has been no decrease in the use of insecticide-treated nets, uptake of other childhood vaccinations or health seeking behavior for febrile illness.
- High impact in real-life childhood vaccination settings: Significant reduction (30%) in deadly severe malaria, even when introduced in areas where insecticide-treated nets are widely used and there is good access to diagnosis and treatment.
- Highly cost-effective: Modelling estimates that the vaccine is cost effective in areas of moderate to high malaria transmission.
Next steps for the WHO-recommended malaria vaccine will include funding decisions from the global health community for broader rollout, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies.
Financial support
Financing for the pilot programme has been mobilized through an unprecedented collaboration among three key global health funding bodies: Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid.
Note to editors:
- The malaria vaccine, RTS,S, acts against P. falciparum, the most deadly malaria parasite globally, and the most prevalent in Africa.
- The Malaria Vaccine Implementation Programme is generating evidence and experience on the feasibility, impact and safety of the RTS,S malaria vaccine in real-life, routine settings in selected areas of Ghana, Kenya and Malawi.
- Pilot malaria vaccine introductions are led by the Ministries of Health of Ghana, Kenya and Malawi.
- The pilot programme will continue in the 3 pilot countries to understand the added value of the 4th vaccine dose, and to measure longer-term impact on child deaths.
- The Malaria Vaccine Implementation Programme is coordinated by WHO and supported by in-country and international partners, including PATH, UNICEF and GSK, which is donating up to 10 million doses of the vaccine for the pilot.
- The RTS,S malaria vaccine is the result of 30 years of research and development by GSK and through a partnership with PATH, with support from a network of African research centres.
- The Bill & Melinda Gates Foundation provided catalytic funding for late-stage development of RTS,S between 2001 and 2015.